Share Important Moment of MileCell Bio with You
2026.01.26
Adipose tissue-derived mesenchymal stem cells (AD-MSCs) have become increasingly popular in regenerative medicine and translational research due to their abundant availability, ease of isolation, and robust regenerative capabilities. However, cryopreservation remains a critical challenge—traditional freezing media relying on DMSO often lead to reduced post-thaw viability, altered cell function, and safety risks that hinder clinical application. Kryogene® Cell Freezing Media-DMSO Free addresses these pain points head-on, delivering a non-toxic, regulatory-compliant solution that preserves AD-MSCs viability, identity, and differentiation potential, as confirmed by rigorous in vitro testing.
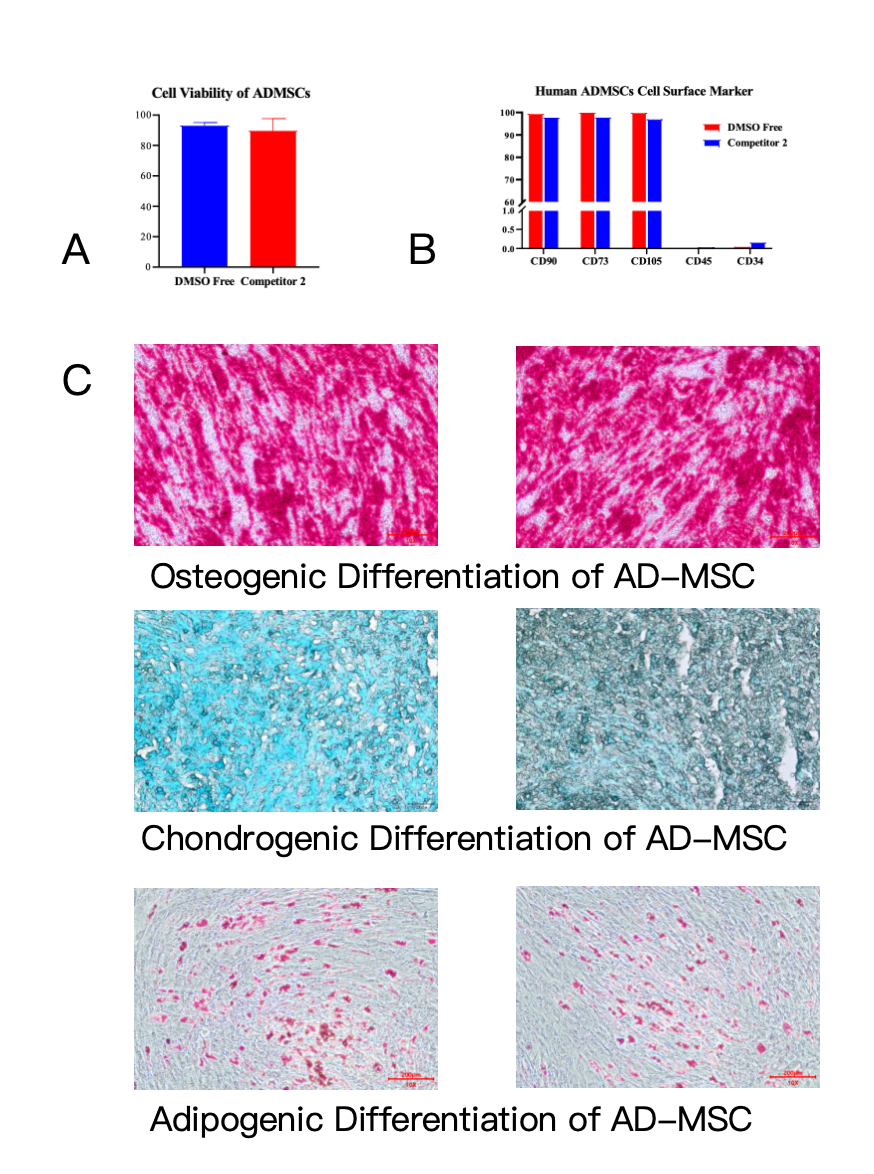
Figure 1 (A) highlights a key advantage of Kryogene® DMSO-Free: its ability to maintain exceptional post-thaw cell viability of AD-MSCs, on par with leading competitive products, without the use of DMSO. This is pivotal for AD-MSCs applications, as adipose-derived cells are often used in large quantities for therapies such as soft tissue repair and immunomodulation—any loss of viability can compromise treatment outcomes. Unlike DMSO-containing media, Kryogene® eliminates cytotoxicity and the need for post-thaw washing, simplifying workflows while protecting cell health.
Cell surface marker expression is a critical benchmark for AD-MSCs quality, as it confirms the cells’ identity and therapeutic relevance. As shown in Figure 1 (B), AD-MSCs cryopreserved with Kryogene® DMSO-Free maintain stable expression of canonical positive markers (CD90, CD73, CD105) and low expression of negative markers (CD45, CD34), adhering to strict international criteria for mesenchymal stem cells. This consistency is essential for research reproducibility and clinical compliance, ensuring that AD-MSCs used in trials or treatments are homogeneous and functionally reliable.
The true measure of a cryopreservation medium lies in its ability to preserve cellular functionality—and Kryogene® excels here. Figure 1 (C) demonstrates that post-thaw AD-MSCs preserved with Kryogene® DMSO-Free retain strong adipogenic, osteogenic, and chondrogenic differentiation capacity. This means the cells can still differentiate into specialized cell types critical for regenerative applications, from bone and cartilage tissue engineering to adipose tissue reconstruction. This functional retention is attributed to Kryogene®’s optimized, serum-free, and animal-derived component-free formulation, which minimizes cellular stress during freezing and thawing.
Kryogene® Cell Freezing Media-DMSO Free is engineered to meet the unique needs of AD-MSCs research and clinical translation. Its cGMP-compliant manufacturing process, using USP-grade raw materials, ensures alignment with global regulatory requirements for advanced cell therapies—reducing regulatory delays and accelerating time-to-market for cell-based products. The single-use manufacturing system eliminates cross-contamination risks, while stringent quality control ensures endotoxin levels ≤ 0.5 EU/mL, meeting the highest safety standards for clinical use.
For biotech companies, research institutions, and clinicians working with AD-MSCs, Kryogene® offers a transformative solution: a DMSO-free medium that doesn’t sacrifice performance for safety. By preserving viability, identity, and differentiation potential, it ensures that AD-MSCs retain their therapeutic value through cryopreservation, enabling more reliable research outcomes and safer clinical applications. Whether used in preclinical studies, clinical trials, or commercial cell therapy products, Kryogene® DMSO-Free is the gold standard for AD-MSCs cryopreservation—empowering the next generation of regenerative medicine.