Share Important Moment of MileCell Bio with You
2026.01.26
In the rapidly evolving field of regenerative medicine, umbilical cord-derived mesenchymal stem cells (UC-MSCs) have emerged as a cornerstone of advanced cell therapies, thanks to their exceptional immunomodulatory properties, high proliferation potential, and multi-lineage differentiation capacity. However, a critical bottleneck in unlocking their full clinical and research potential lies in cryopreservation—traditional DMSO-containing freezing media often introduce cytotoxicity, regulatory hurdles, and post-thaw functional degradation, undermining the reliability of UC-MSCs for translational applications. This is where Kryogene® Cell Freezing Media-DMSO Free steps in, setting a new standard for safe, effective, and compliant UC-MSCs cryopreservation, as validated by rigorous in vitro functional assessments.
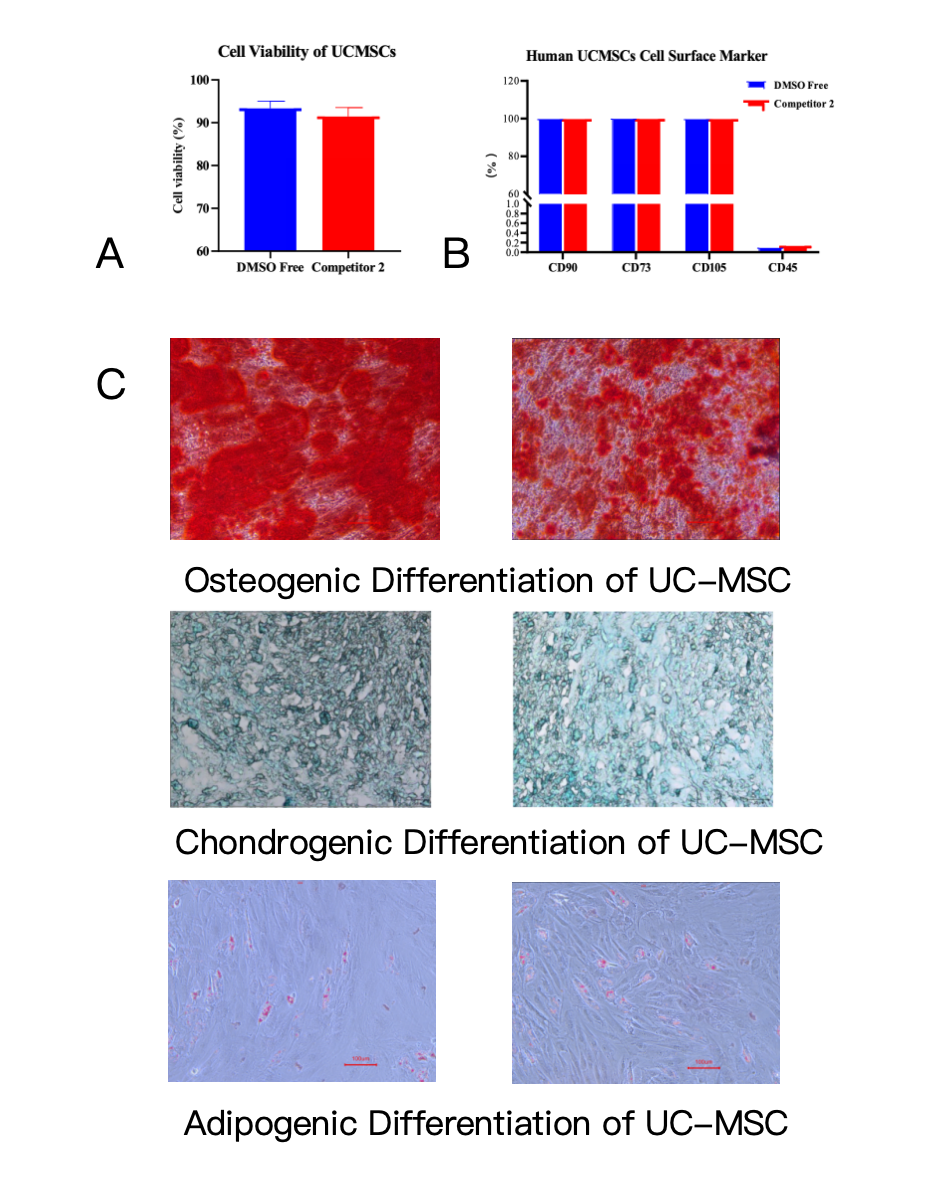
As demonstrated in Figure 1 (A), post-thaw cell viability of UC-MSCs preserved with Kryogene® DMSO-Free is comparable to leading competitive products, even without the use of DMSO. This is a game-changer for researchers and clinicians, as DMSO—a common component in traditional cryopreservation media—poses well-documented risks, including cell membrane damage, immune reactions in clinical settings, and the need for time-consuming post-thaw clearance. Kryogene®’s optimized formulation eliminates these concerns while maintaining robust cell survival, ensuring that UC-MSCs retain their core biological integrity after freezing and thawing.
Beyond viability, the preservation of cell surface markers is critical for confirming UC-MSCs identity and therapeutic potential. Figure 1 (B) shows that UC-MSCs cryopreserved with Kryogene® DMSO-Free exhibit stable expression of key positive markers (CD90, CD73, CD105) and minimal expression of negative markers (CD45, CD34)—a profile that aligns with international standards for mesenchymal stem cells. This consistency is vital for research reproducibility and clinical compliance, as deviant surface marker expression can compromise the efficacy of cell-based treatments.
Most importantly, Kryogene® DMSO-Free preserves the multi-lineage differentiation capacity of UC-MSCs, the defining feature that makes them invaluable for regenerative medicine. Figure 1 (C) validates that post-thaw UC-MSCs retain strong adipogenic, osteogenic, and chondrogenic differentiation potential—enabling their use in applications ranging from bone and cartilage repair to adipose tissue regeneration. This functional retention is made possible by Kryogene®’s clinical-grade formulation, which is free of serum and animal-derived components, minimizing batch-to-batch variability and clinical risks.
Kryogene® Cell Freezing Media-DMSO Free is not just a DMSO alternative—it’s a comprehensive solution designed for the demands of modern cell therapy. Manufactured under current Good Manufacturing Practice (cGMP) standards with USP-compliant raw materials, it meets global regulatory guidelines, streamlining the path from preclinical research to clinical translation. Its single-use manufacturing process eliminates cross-contamination risks, while strict quality control ensures endotoxin levels ≤ 0.5 EU/mL, guaranteeing product purity and safety.
For researchers, biotech companies, and clinical institutions invested in UC-MSCs-based therapies, Kryogene® DMSO-Free offers a dual advantage: enhanced safety and uncompromised functionality. By removing DMSO and animal-derived components, it addresses the most pressing regulatory and toxicological concerns, while its data-proven performance ensures that UC-MSCs retain their therapeutic potential through cryopreservation. In an industry where every cell counts, Kryogene® is the trusted partner for advancing cell therapies with confidence.