
Share Important Moment of MileCell Bio with You
2025.08.16
Cryopreserved cynomolgus monkey (Macaca fascicularis) hepatocytes, with their high genetic homology and functional similarity to human liver cells, serve as the preferred in vitro model for studying liver-targeted drug delivery. Their core advantage lies in the high expression of the asialoglycoprotein receptor (ASGPR), which mediates endocytosis via specific binding to GalNAc-modified ligands. ASGPR is a pivotal target for GalNAc-siRNA, protein-based therapeutics, and nanoparticle delivery systems. Its liver-specificity, high affinity, and efficient recycling capacity form the molecular foundation for reducing systemic toxicity and enhancing targeting efficiency, while also offering novel strategies for metabolic diseases, viral hepatitis, and other liver-related therapies.
ASGPR: The Key Molecular Target for Liver-Targeted Therapies
ASGPR is an endocytic receptor exclusively expressed on hepatocyte membranes, facilitating high-efficiency uptake of galactose- or GalNAc-terminated ligands via clathrin-dependent pathways. Its advantages as a liver-targeting platform include:
l Tissue specificity: Predominantly expressed in hepatocytes, minimizing off-target organ uptake.
l High affinity: Nanomolar-level binding to GalNAc-conjugated ligands ensures precise targeting.
l Dynamic endocytosis: Rapid internalization and recycling of receptor-ligand complexes support high-dose delivery without saturation.
These properties make ASGPR the gold-standard target for GalNAc-siRNA, protein drugs, and nanocarriers. Studies confirm that ASGPR-mediated delivery significantly enhances hepatic drug accumulation while reducing systemic toxicity, revolutionizing treatments for metabolic disorders, genetic liver diseases, and viral hepatitis.
Milecell Bio Provides Cryopreserved Cynomolgus Hepatocytes with High ASGPR Expression
Validation of ASGPR Expression Dynamics and Membrane Localization
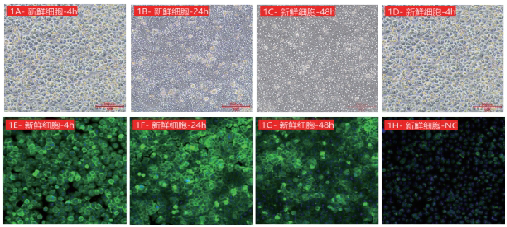
Figure 1: Dynamic analysis of ASGPR expression in fresh cynomolgus monkey hepatocytes. 1A-1D show the adherent status of hepatocytes at different time points under bright field observation; 1E-1H show the results of immunofluorescence expression detection, with 1H being the negative control. Green fluorescence represents ASGPR, and blue fluorescence represents DAPI nuclear staining.
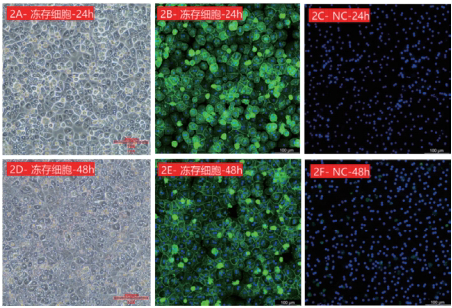
Figure 2: Dynamic analysis of ASGPR expression in cryopreserved cynomolgus monkey hepatocytes. 2A and 2D show the adherent morphology under bright field observation at 24h and 48h, respectively; 2B and 2E show the detection of ASGPR expression by immunofluorescence at 24h and 48h, respectively; 2C and 2F are negative controls. Green fluorescence indicates ASGPR, and blue fluorescence indicates DAPI nuclear staining.
Fresh cynomolgus monkey hepatocytes maintained high ASGPR expression within 4-48 hours of adherent culture (1E-1G), with the peak at 24 hours (1F). Cryopreserved cynomolgus monkey hepatocytes showed no significant differences from fresh cells in terms of adhesion efficiency (1B, 2A), dynamic ASGPR expression (2B, 2E) and membrane localization characteristics.
Three Key Advantages of Milecell Bio’s Cryopreservation System:
l Long-term stability: Eliminates donor variability and batch fluctuations.
l Pre-validated functionality: Standardized ASGPR expression and metabolic activity.
l Experimental flexibility: On-demand thawing for complex study designs.
Product Highlights
l ASGPR membrane localization (≥90% positivity)
l Functional CYP3A/2C/2B/1A enzyme activity
l Post-thaw viability ≥90%
Model Superiority
l Physiological relevance: Cryopreserved cells mirror fresh cells in ASGPR expression and adhesion, ensuring reliable in vitro modeling.
l Cross-species translation: >90% homology in ASGPR/CYP profiles between cynomolgus and human hepatocytes bridges preclinical-to-clinical gaps.
Applications
l Targeted drug development: ASGPR-driven uptake studies for GalNAc-conjugated therapeutics.
l Metabolism/toxicity prediction: CYP enzyme activity models drug interactions and hepatotoxicity thresholds.
l Translational research: Cynomolgus models address rodent-human disparities.
References
[1] D'Souza, A. A., & Devarajan, P. V. (2015). Asialoglycoprotein receptor mediated hepatocyte targeting—strategies and applications. Journal of Controlled Release, 203, 126-139. DOI
[2] Cui, H., et al. (2021). Liver-targeted delivery of oligonucleotides with N-acetylgalactosamine conjugation. ACS Omega, 6(25), 16259-16265. DOI
[3] Debacker, A. J., et al. (2020). Delivery of oligonucleotides to the liver with GalNAc: From research to registered therapeutic drug. Molecular Therapy, 28(8), 1759-1771. DOI
Note: Formatting adheres to the original structure, with embedded figures placed adjacent to their contextual descriptions. Key terms (e.g., ASGPR, GalNAc) are consistently highlighted for emphasis.
