
Share Important Moment of MileCell Bio with You
2025.02.14
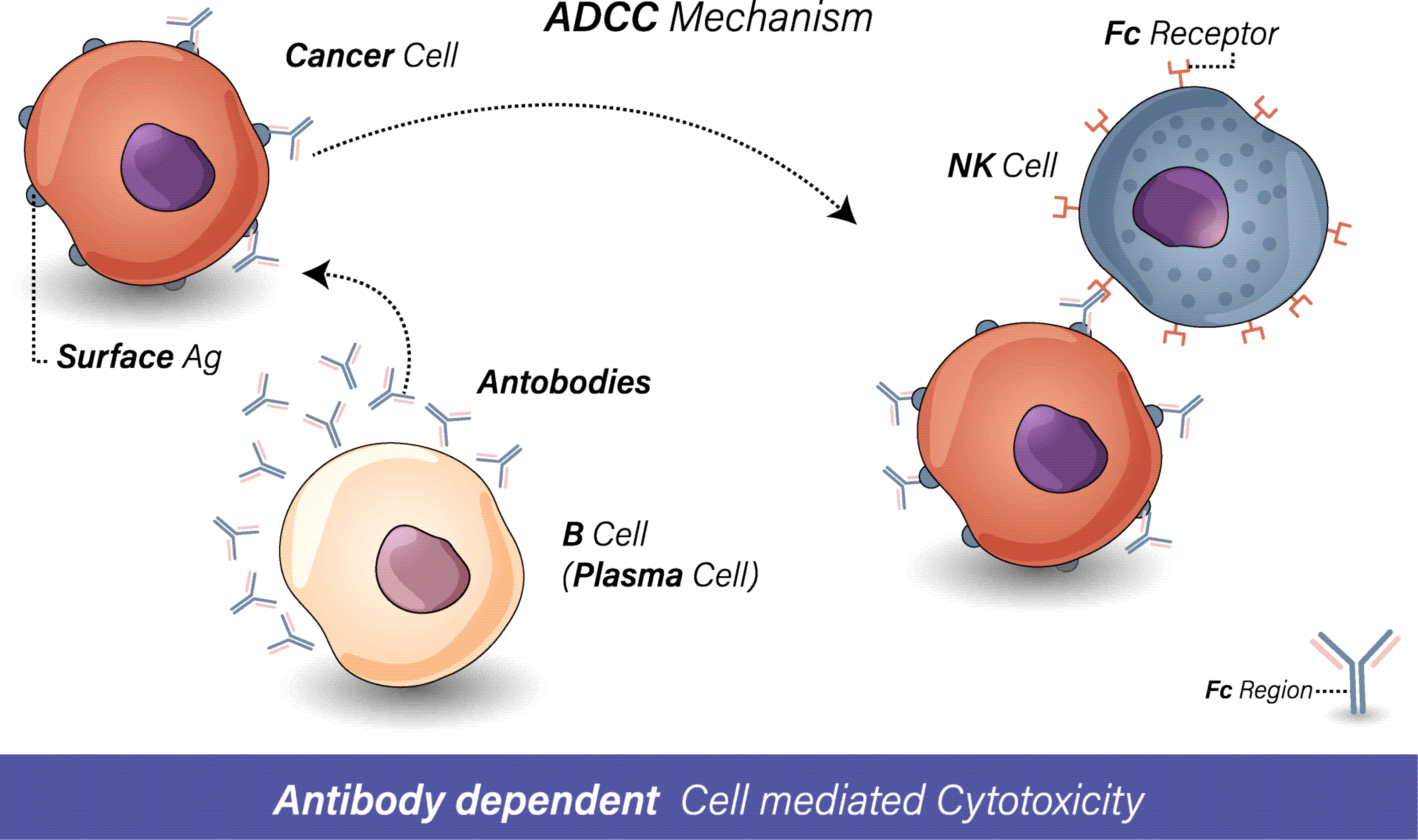
Antibody-dependent cellular cytotoxicity (ADCC) is a critical immune mechanism that bridges adaptive and innate immunity, enabling antibodies to direct immune cells to destroy pathogens or diseased cells. This process plays a pivotal role in the efficacy of many therapeutic antibodies, particularly in oncology, infectious diseases, and autoimmune disorders. Understanding ADCC’s molecular basis and optimizing its experimental evaluation are essential for advancing targeted therapies.
ADCC begins when the Fab region of an IgG antibody binds to a specific antigen on the surface of a target cell (e.g., a cancer cell or virus-infected cell). Simultaneously, the antibody’s Fc region engages with activating Fc gamma receptors (FcγRs), primarily FcγRIIIa (CD16), expressed on effector immune cells like natural killer (NK) cells, macrophages, or neutrophils. This dual interaction creates a lethal synapse:
Target Recognition: Antibody-antigen binding marks the cell for destruction.
Effector Activation: FcγRIIIa crosslinking triggers effector cells to release cytotoxic granules (e.g., perforin, granzymes) or induce apoptosis via death receptors (e.g., Fas/FasL).
Target Elimination: Pore-forming perforin allows granzymes to enter the target cell, activating caspase cascades that dismantle cellular components.
NK cells are the primary mediators of ADCC due to their high CD16 expression and rapid response capability. However, their activity depends on the balance between activating (CD16, NKG2D) and inhibitory receptors (KIRs), which prevent attacks on healthy "self" cells.
ADCC is a double-edged sword in antibody design. While enhancing it can boost therapeutic potency, uncontrolled activation risks off-target toxicity. Key applications include:
1. Cancer Immunotherapy
Anti-CD20 antibodies (e.g., Rituximab): Eliminate B-cell malignancies by recruiting NK cells to CD20+ lymphoma cells.
Anti-HER2 antibodies (e.g., Trastuzumab): Target HER2-overexpressing breast cancer cells, with ADCC contributing to ~30% of their clinical efficacy.
2. Antiviral Therapies
Antibodies against HIV, influenza, or SARS-CoV-2 leverage ADCC to clear infected cells. For example, broadly neutralizing antibodies (bNAbs) against HIV’s envelope glycoproteins rely on ADCC to suppress viral reservoirs.
3. Antibody Engineering
Modifying an antibody’s Fc domain can fine-tune ADCC:
Glycoengineering: Reducing fucose content in the Fc’s N-linked glycan (e.g., Obinutuzumab) enhances CD16 binding, boosting NK cell activation.
Fc Mutagenesis: Introducing S239D/I332E mutations increases FcγRIIIa affinity, amplifying cytotoxicity.
Reliable ADCC evaluation requires meticulous experimental design:
1. Effector Cell Selection
Primary PBMCs: Fresh or thawed peripheral blood mononuclear cells (PBMCs) provide physiologically relevant NK cells but face donor variability.
Engineered Cell Lines: CD16-transfected NK-92 or Jurkat cells offer consistency but lack primary cell complexity.
2. Target Cell Models
Cell Lines: Engineered to overexpress target antigens (e.g., CHO cells with CD20).
Primary Cells: Patient-derived tumor cells better mimic in vivo conditions but are harder to standardize.
3. Functional Readouts
Chromium-51 Release: Gold standard for quantifying cytotoxicity but involves radioactivity.
Flow Cytometry: Measures target cell apoptosis via Annexin V/PI staining or CFSE-labeled target loss.
Luminescent Assays (e.g., BioLucx): Detect granzyme B activity or caspase activation in real time.
4. Controls
Isotype-matched antibodies to rule out nonspecific killing.
Fc-blocking reagents (e.g., IVIG) to confirm ADCC specificity.
Emerging techniques like single-cell RNA sequencing and high-content imaging are unraveling ADCC’s heterogeneity—such as why certain NK cell subsets dominate cytotoxicity or how tumor microenvironments suppress ADCC. Additionally, bispecific antibodies (e.g., CD16xCD30) are being designed to forcibly recruit NK cells to resistant tumors.
Conclusion
ADCC remains a cornerstone of antibody therapeutics, balancing precision and immune activation. By deepening our understanding of its mechanisms and refining experimental models, researchers can develop smarter therapies that harness the immune system’s killer instinct—without collateral damage.
