
Share Important Moment of MileCell Bio with You
2025.01.10
Product Information
Kryogene™ Cell Freezing Media - CGT (FDA MasterFile) is a series of highly efficient, safe, and easy-to-use cryopreservation media optimized for the special requirements of a variety of cell and gene therapy drugs, ensuring that cells maintain optimal viability and functionality during the freezing, storage, and thawing.
Product Feature
DMSO Pre-Formulated
Serum-Free
Protein-Free
USP Components
No Ingredients
of Animal Origin
GMP-compliant Production
Sterility
Ultra low endotoxin
Cell Cryopreservation
Release Testing
Product Information

Human Immune Cell Subtype:
Cryopreserved hCD34+ Cell
Functional Tests
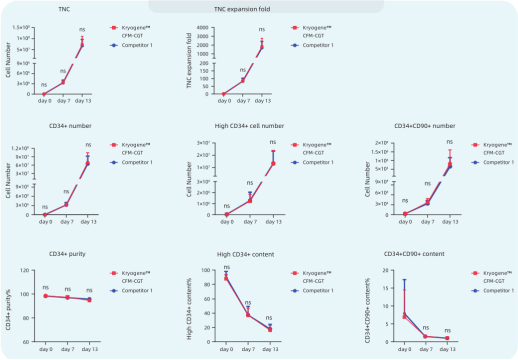
Fig 1. Human Mobilized Peripheral Blood-derived hCD34+ (Donor 1) and Umbilical Cord Blood-derived hCD34+ (Donor 2, Donor 3) were isolated and cryopreserved in Kryogene™ CFM-CGT, competitor 1, respectively. After 3 months, the cells were thawed and cultured using commercial expansion kits to compare the proliferative capacity. The number of TNC (Total nucleated cell) with expansion multiplicity was calculated on day 7 and day 13, while HSPC (CD34+, High CD34+, CD34+/CD90+) expansion percentages were analyzed via flow cytometry.
Multi-Donor Cryopreserved PBMC
Functional Tests
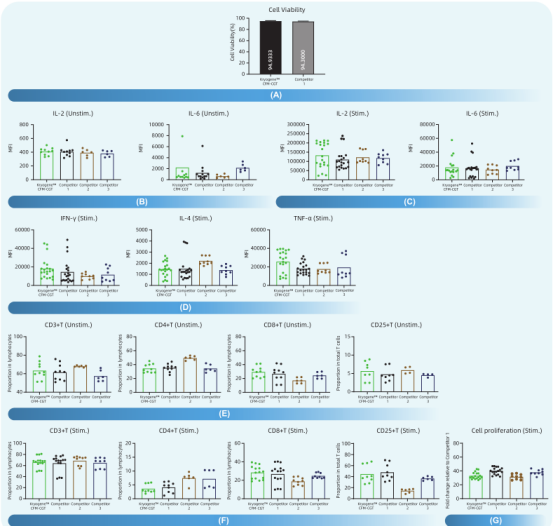
Fig 2. Human PBMCs derived from multiple donors were cryopreserved using Kryogene™ CFM-CGT, as well as three competitive freezing media. After thawing, cell viability and functionality were assessed. PBMCs were stimulated with PMA-Inomycin for activation, and cytokine release was measured using the CBA method. T-cell activation and phenotypes were analyzed via flow cytometry. PBMCs labeled with Celltrace were stimulated with CD3/28 beads to assess cell proliferative activity using flow cytometry.
(A) Evaluation of post-thaw cell viability
(B) Unactivated background cytokine release
(C) Detection of cytokine release following activation
(D) Analysis of cytokine release post-activation
(E) Assessment of the proportion of inactivated cell subtypes
(F) Determination of the proportion of activated cell subtypes
(G) Evalution of PBMC proliferative activity
