Share Important Moment of MileCell Bio with You
2024.07.15
MileCell’s Liver S9 Fractions in ADME/Tox Tools for Drug Preclinical Studies
Liver fractions play a crucial role in ADME (Absorption, Distribution, Metabolism, and Excretion) and toxicology (Tox) studies during the preclinical phase of drug development. These fractions, including microsomes, cytosol, and S9 fractions, are derived from liver tissue and are essential for evaluating the metabolic fate of drug candidates. Here, we focus on the characterization and applications of animal liver S9 fractions and compare them to microsomes.
Liver S9 Fractions
Liver S9 fractions are prepared by centrifuging liver homogenates at 9,000 x g for 10 minutes. The supernatant, a combination of unfractionated microsomes and cytosol, is rich in a diverse array of drug-metabolizing enzymes and is widely used as an ideal test system for various in vitro ADME studies, particularly those investigating both phase I and phase II drug metabolism.
・Enzymes Present: Phase I (CYPs, FMOs) and phase II (GSTs, SULTs, NATs)
・Metabolic Reactions: Both phase I and phase II metabolism
・Applications: Metabolic studies, stability screening, toxicological assessments, species comparison
We provide animal liver S9 products from different species, with donor pools matched for inter-study consistency. This facilitates easier correlation between test systems in preclinical drug assays. Our S9 options are optimized to offer the following benefits:
・Matching microsomes and S9 across most donor pools・Large donor pools to reduce lot-to-lot variation and ensure long-term availability
・Thorough characterization using validated LC-MS/MS methods
・Thorough characterization using validated LC-MS/MS methods
Characterization of Animal Liver S9 Fractions
Our animal liver S9 fractions are characterized by their enzymatic activity, protein content, and ability to metabolize various substrates. These fractions are obtained from different species, such as rats, mice and dogs, providing comparative insights into interspecies differences in drug metabolism. Our liver S9 fractions demonstrate excellent quality compared to well-known branded products, as evidenced by comparative metabolic stability studies.
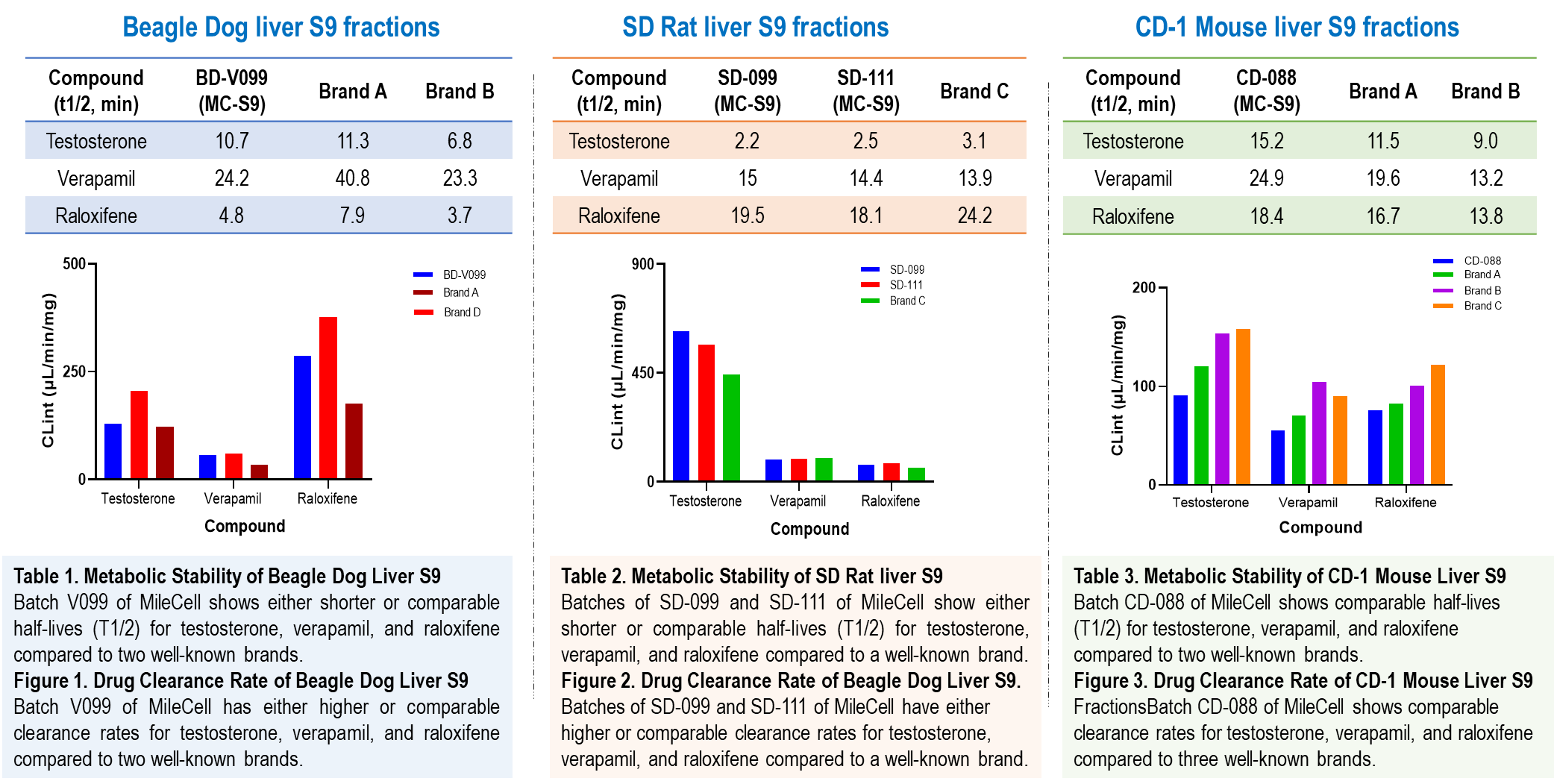
Applications of Animal Liver S9 Fractions
1.Metabolic Stability Studies: Assessing the half-life of drug candidates by determining their metabolic rate.
2.Identification of Metabolites: Detecting both phase I and phase II metabolites to understand the metabolic pathways.
3.Toxicological Assessments: Evaluating the formation of potentially toxic metabolites and reactive intermediates.
4.Drug-Drug Interaction Studies: Investigating the potential for drug candidates to inhibit or induce metabolic enzymes.
5.Interspecies Comparison: Comparing metabolic profiles across different animal species to predict human metabolism.
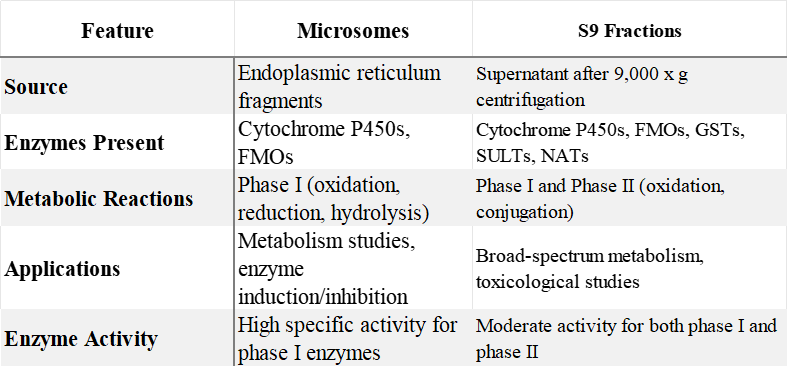
Comparison Table: Microsomes vs. S9 Fractions
Conclusion
Liver fractions such as microsomes and S9 are indispensable tools in the ADME/Tox evaluation of drug candidates. Microsomes are primarily used for phase I metabolic reactions, while S9 fractions, containing both phase I and phase II enzymes, offer a comprehensive approach to metabolism and toxicology studies. Understanding the differences between these fractions and their specific applications is crucial for the effective design of preclinical studies and the successful development of new pharmaceuticals.
MileCell’s liver S9 products are now available for your broad-spectrum metabolism studies:
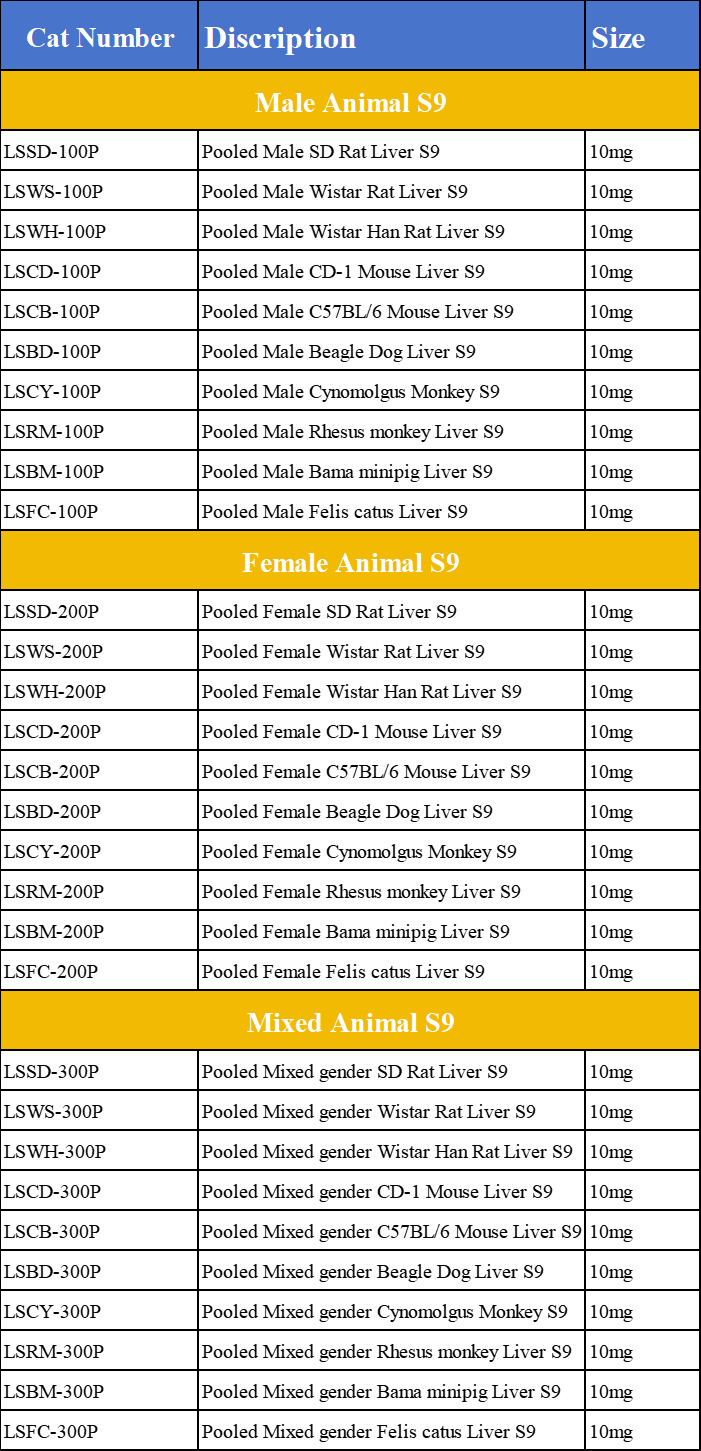
MC Liver S9 Product List